A Chemical Shell Game: How DuPont Concealed the Dangers of the New Teflon Toxin
HEALTH, 7 Mar 2016
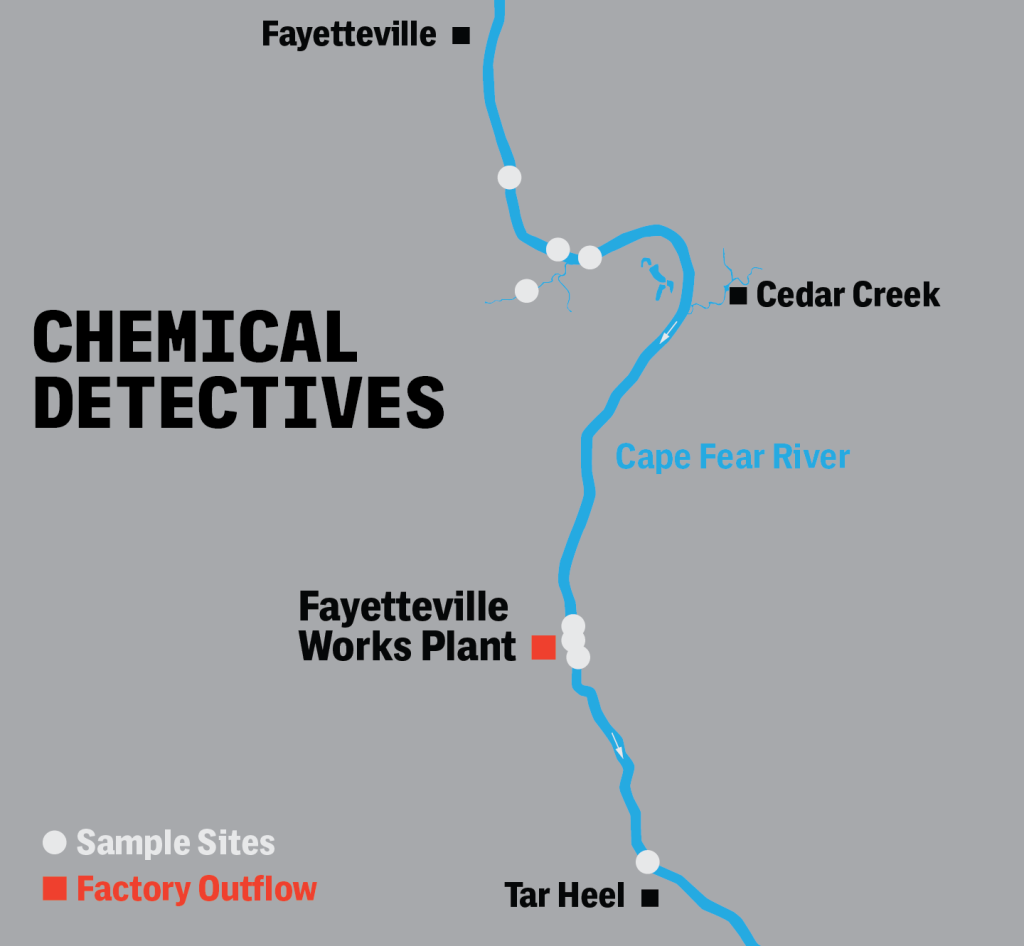
3 Mar 2016 – Mark Strynar and Andrew Lindstrom walked down the muddy bank of the Cape Fear River toward the water, sampling equipment in hand. It was the summer of 2012, and the scientists, who both work for the Environmental Protection Agency, were taking the first steps in what would be more than two years of detective work. The Cape Fear winds its way for over 200 miles through North Carolina before flowing into the Atlantic, but Strynar and Lindstrom were focused on a 20-mile stretch that runs from a boat dock outside Fayetteville south to the little town of Tar Heel. About halfway between the two points, on the western bank of the river, sits a large plant built by DuPont.
Fayetteville Works, as the sprawling site is called, previously manufactured C8, a chemical that DuPont used for more than 50 years to make Teflon and other products. After a massive class-action lawsuit revealed evidence of C8’s links to cancer and other diseases, DuPont agreed in a deal with the EPA to phase out its use of the chemical. But Strynar and Lindstrom were among many scientists who feared that DuPont and the other companies that used C8 might have swapped it out for similar compounds with similar problems. To see if they were right — and whether any of these replacements might have ended up in the river — they took water samples from the Cape Fear, some upstream the plant, others from points below its outflow.
Perfluorooctanoic acid, commonly known as PFOA or C8, is a “perfluorinated” chemical, which means that its base includes carbon chains attached to fluorine atoms. Because the fluorine-carbon bond is one of the strongest in chemistry, these compounds are incredibly stable, which makes them useful in industry. But that stability also makes them endure in the environment. Indeed, C8, which has recently been detected in upstate New York, in Vermont, and in Michigan’s Flint River, among other places, is expected to remain on the earth long after humans are extinct. And evidence suggests that many of its replacements are just as persistent.
The potential permanence of the problem was only one reason the EPA team was mucking around on the banks of the Cape Fear River. There were short-term dangers, too. Strynar and Lindstrom knew well that the Cape Fear is a source of drinking water and that if perfluorinated chemicals — known as PFCs — had contaminated the river, they would soon make their way into human bodies. Strynar had spent eight years documenting the presence of these molecules in fish, food, air, house dust, and humans. Lindstrom, an expert on measuring PFCs in the environment who has worked for the EPA for more than two decades, had also been documenting the steady proliferation of the chemicals. Both knew that the potential for contamination around the plant was great, because C8 had spread into the water around many of the facilities that made and used it, including plants in West Virginia, Minnesota, New Jersey, Alabama, Germany, and Japan. According to data from the Centers for Disease Control, 99.7 percent of Americans already had C8 in their blood.
What Lindstrom and Strynar didn’t know was exactly what DuPont had used to replace C8 and whether it was escaping the plant. The river water was their key to finding out. By comparing the samples from above and below the plant’s outflow, they could determine which chemicals may have entered the river at that point.
Strategic sampling was the easy part. Figuring out the exact chemical structure of those molecules would require more ingenuity. Ultimately, it would take a team of 10 scientists from five different institutions more than a year to figure out the structure of the PFCs they found in the river — using a mass spectrometer, which produced spiky graphs depicting the exact weight and features of each molecule, software that uses the masses of compounds to generate likely chemical formulas, and painstaking searches of chemical databases and public records for descriptions of new PFCs to compare against their findings. Altogether, the scientists found 12 new PFCs, including one discovered in the files of the West Virginia Department of Environmental Protection, which in 2011 approved DuPont’s use of a C8 replacement at its Washington Works factory in Parkersburg. That was the same facility that had caused massive C8 contamination of drinking water linked to severe health problems among the local population.
After analyzing the molecules, Strynar and Lindstrom concluded that “a new generation of replacement compounds is now out in the environment,” they wrote in response to questions from The Intercept. These new chemicals likely had “the same chemical performance properties” as the older generation of PFCs, like C8. “This would also suggest,” they wrote, “that their toxicity and environmental persistence are likely to be similar as well.”
17,585 Secret Chemicals
When companies want to begin making and selling a new chemical, they are required to file a written notice with the EPA. But current regulations do not mandate that any particular health or safety studies be performed, and according to a 2007 report from the EPA, only 15 percent of new chemical notices contain any information about the materials’ impact on health. Moreover, chemical manufacturers are permitted to claim that various parts of the information they give the EPA are “confidential business information,” or CBI. About 95 percent of new chemical notifications, according to a 2005 Government Accountability Office report, include information that is protected as a trade secret, a figure the EPA confirmed as still “generally accurate.”
Even the very name and structure of a chemical, which are essential to tracking its presence in food, water, and the rest of the environment and determining how it affects humans, can be claimed as CBI. The 12 chemicals Strynar and Lindstrom’s team painstakingly identified are just the tip of a mysterious and dangerous iceberg. Manufacturers have used the CBI shield to withhold the names and identities of 17,585 of the chemicals now registered with the EPA.
The allowance for certain confidentiality claims, which is written into the law, is based on the idea that if companies are forced to reveal the exact nature of a chemical, other companies will be able to duplicate it, depriving the original manufacturer of the opportunity to profit from its research and development investment. In response to past criticism of CBI claims, the American Chemistry Council has said that “balanced confidentiality laws help protect the trade secrets that foster innovation and create jobs.”
But claiming information as CBI means that it’s not only withheld from competitors within industry but also from the general public, manufacturers who use the chemicals in their products, independent scientists who study the impact of these substances on humans and the environment, and most EPA staff, only a fraction of whom have CBI clearance.
“CBI hinders our ability to capture emerging pollutants and make sure the public is safe,” said David Andrews, senior scientist at the Environmental Working Group, whose 2009 report publicly raised the problems posed by the growing list of secret chemicals. “Scientists can’t search for contaminants if they don’t know what they’re looking for.”
The secrecy surrounding DuPont’s C8 replacement, which is sold under the commercial name GenX, left Strynar and Lindstrom in a bizarre situation. Although they work for the EPA’s National Exposure Research Lab, they didn’t have access to all the information they needed to determine whether people were being exposed to the chemical and, if so, whether that exposure posed an environmental risk. They might have applied for CBI clearance, but because those privy to such business secrets are by law forbidden from sharing them, they wouldn’t have been able to reveal what they learned. Compounding the absurdity of their situation, a recent records search has revealed that although the chemical identity of the replacement was initially shielded as CBI, DuPont had declassified it by 2011. As a result, its generic identification number was switched to a traceable number, and information about the chemical was theoretically public. But because there had been no announcement of the declassification and no publication of the traceable number until after Strynar and Lindstrom began their research, no one — including the two EPA scientists — was able to access information about it. And so they had to spend many months and many taxpayer dollars sleuthing out information that was readily available to some of their colleagues within the EPA.
As it turned out, GenX was present in the river.
Sanitized Documents
After a manufacturer tells the EPA about a new chemical it would like to introduce, the agency has 90 days to respond. While it most often simply accepts these new creations and rarely forbids companies from bringing them to market, in about 10 percent of new chemical applications since 1979 and about 40 percent of the notices submitted last year, the agency gave its version of a yellow light, requiring some sort of testing or restrictions on the production of the substance. These requests often take the form of consent orders. Publicly available versions of these documents are often riddled with redactions meant to protect confidential trade secrets.
For instance, a consent order for three PFCs issued in 2006, after the phase-out of C8 was announced, bears the stamp, “EPA SANITIZED,” and notes that critical details such as “company identity, specific chemical identities, production volumes, manufacturing process, processing and use information, and other information” have been scrubbed from it on the grounds of CBI.
The absence of this information makes what does come to light in the rest of the document particularly disturbing. The consent order for the three chemicals acknowledges that the EPA is concerned they “could cause lung effects” and notes that they may degrade into substances that “will persist in the environment, could bioaccumulate or biomagnify, and could be toxic (“PBT”) to people, wild mammals and birds.” These factors taken together, the consent order concludes, “raise concerns for potential adverse chronic effects in humans and wildlife.”
Despite these concerns, the EPA allowed the three replacement chemicals to enter the market in 2006 with the provision that the company perform reproductive, toxicity, and carcinogenicity tests of the chemicals’ effects on rats. Because the testing was required only if the company made or imported more than a certain amount of the chemicals — and because that “trigger amount” was withheld as CBI — it’s unclear if the company ever reached that limit or if the testing was ever done.
When asked about this document, the EPA provided the following response: “Based on concerns raised during the review of three alternative chemicals, a consent order was put in place (and later modified) that requires certain fate testing (i.e., hydrolysis, photolysis and biodegradation studies) to be completed in 2016 and 2017. The data will allow us to better understand the degradation rate of the chemicals.”

The flame retardant chemical Firemaster 550 is made at the Chemtura-owned Great Lakes Solutions plant in El Dorado, Ark., as seen May 2, 2012.
Photo: Alex Garcia/Chicago Tribune/Getty Images
Regrettable Substitutions
Several dangerous chemicals have been replaced by what environmentalists call “regrettable substitutions,” molecules that are often just slightly tweaked versions of the originals and pose similar problems. After PCBs were associated with health problems, including lowered immune response and developmental issues, the chemicals that replaced them also proved to be toxic. And in perhaps the most notorious recent example, bisphenol S (BPS), an additive to plastic used for water bottles and sippy cups, turned out to have many of the same dangerous characteristics as the close chemical cousin it replaced, bisphenol A (BPA).
But while PCBs had only a handful of replacements, and BPA had one primary substitute, the phasing out of PFOA and other PFCs based on 8-carbon chains has led to the introduction of a much larger number of chemicals.
Between 2006 and 2011, after manufacturers agreed to phase out longer-chain PFCs, chemical companies notified the EPA of their intent to introduce some 150 chemicals to replace them, according to research conducted by the Environmental Defense Fund in 2012. At least 125 of those chemical names were claimed as confidential.
Over the past decade, the EPA has reviewed more than 300 proposed alternatives to C8, according to a written response the agency provided to questions from The Intercept. Of those applications, 0.9 percent were not accepted; 67.1 percent were subject to consent orders, which often require additional testing of the chemical; and 18.5 percent were withdrawn by the submitter, “often in the face of regulatory action.”
The manufacture of just one of these compounds can result in many byproducts, which themselves can be dangerous. Several of the 12 PFCs Strynar and Lindstrom found in the Cape Fear River may have been created through the process of making GenX.
While Strynar and Lindstrom were searching the river for signs of DuPont’s C8 replacement, a PhD student in Europe confirmed the chemical structure of GenX in a surprising place. Zhanyun Wang, whose dissertation focused on PFCs, was at a conference in Munich in 2012 when he met a DuPont employee who told him that the formula for GenX had been printed in a brochure.
When Wang, now an environmental scientist who spends much of his professional life tracking down and sharing hidden information about dangerous chemicals, got home from the conference, he easily found a copy of the brochure on the DuPont website. The formula of GenX — CF3CF2CF2OCF(CF3)COOH.NH3 — was right there on Page 2. He told me he assumed that its publication was a mistake but went ahead and included the formula in a 2013 paper that included a roundup of replacements for long-chain PFCs.
Shortly after his paper came out, Wang ran into some colleagues who worked for DuPont. “They were not happy,” Wang recalled. “But then they found out it was from their documents so there was nothing they could do.”
Confidential A and B
Recently, CBI claims have hobbled the EPA’s efforts to move forward with the regulation of a group of flame retardants known as brominated phthalates clusters (BPCs). These chemicals were introduced to replace older flame retardants that accumulate in humans and the environment, and were banned in some states after being linked to developmental problems, hormone disruption, and cancer.
Like the older flame retardants, BPCs are present in furniture, electronics, and some baby items. Although researchers have only recently begun studying BPCs, they have already raised some of the same red flags, and have linked the newer flame retardants to DNA damage and hormone disruption. Chemtura, one of the companies that made the previous generation of flame retardants, is also producing at least two of these new chemicals and together with two other manufacturers made somewhere between 1 and 10 million pounds of one BPC in 2011, according to the Chemical Data Reporting Database.
In 2013, the EPA began to officially assess the risks posed by BPCs, but in August 2015 it published a document known as a “data needs assessment,” which concluded that the agency still needed more data. The report reveals how much information the flame retardant industry has withheld from the scientific community. Consider two of the chemicals, listed in the August report only as “Confidential A” and “Confidential B.”
The consent order for Confidential A sums up the problem well: As with other consent orders, this document is heavily redacted, with the name of the chemical, its manufacturer, intended uses, and production quantities all withheld as confidential business information. The few details that do emerge are alarming. For instance, the document notes that the chemical raises concerns about “liver and kidney toxicity” and carcinogenicity in humans, as well as toxicity to fish and aquatic life, while also acknowledging that Confidential A will be used in consumer goods and may be “persistent, bioaccumulative, and toxic.”
Nevertheless, the EPA allowed Confidential A to enter the market in 2009 with the provision that the unnamed company perform additional tests to determine whether the chemical affects reproduction and development in rats. These tests, too, were tied to a trigger level that was claimed as a secret. (According to EPA documents, as of August 2015 the trigger level had not been reached.) The consent order for Confidential A also warns the manufacturer against making “predictable or purposeful release” of the chemical into “the waters of the United States.” But, as we know from Strynar and Lindstrom’s experience, the ability to determine whether the chemical has in fact been released hinges on first figuring out what it is.
Perhaps more disturbing is what happened with Confidential B, a chemical that “sailed through the New Chemicals program,” according to comments on the report that the Environmental Defense Fund submitted to the EPA on January 20, 2016. Despite the fact that the unknown chemical is so worrisome that it made it onto a shortlist of chemicals the EPA is investigating, the agency apparently didn’t require its mysterious manufacturer to perform any health testing. In 2015, according to the EPA’s August data needs assessment, Confidential B was grouped among chemicals that were produced in volumes greater than 1 million pounds.
When asked for comment, the EPA noted that it hasn’t received any new test data on Confidential B “because the production volumes are too small” and pointed out that the agency now typically bans the manufacture and import of new BPCs “until up front testing can be conducted and reviewed.” Asked to resolve the inconsistency, the EPA insisted that “for Confidential B, the production value is not greater than 1 million pounds.”
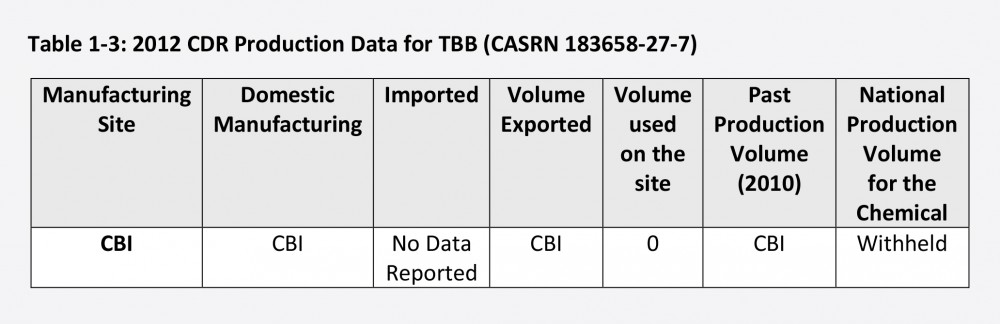
We don’t know much more about the named BPCs. For instance, the production data entry for a chemical known as TBB, one of the seven flame retardants listed in a supplement to the EPA’s report, is essentially devoid of information. The name of the production site, the amount produced domestically, the amount exported, and, as with Confidential A, the amount produced overall, have all been claimed as CBI.
“By calling production volume data CBI, they’re obscuring the extent of how prevalent a chemical is — and how prevalent exposure is,” said Eve Gartner, a staff attorney at Earthjustice, who submitted comments about the BPC data needs assessment to the EPA on behalf of the Natural Resources Defense Council, the Washington Toxics Coalition, and Earthjustice in January. Without this data, said Gartner, the EPA can’t do its job.
“EPA had a legal obligation to find out more about the toxicity of these chemicals and it failed to do that,” said Gartner. “And now it can’t do a risk assessment that might lead to regulation. That means many more years in which people, children, firefighters — everyone — is being exposed to toxic chemicals.” Indeed, while the regulatory process has been stalled, the environmental concentration of two BPCs known as TBB and TBPH has been doubling every year in urban areas and every 1.6 years in rural areas, according to a 2012 article in Environmental Science Technology.
While Gartner admits that some confidentiality claims, including those for the production volume, may fall into a legal gray area, others are plainly violations of the law. The Toxic Substances Control Act (TSCA), which lays the groundwork for chemical regulation, makes it clear that health studies cannot be protected as CBI. Yet, in 2012, Chemtura submitted more than 12 health studies to the EPA that it claimed as CBI.
The EPA did not dispute that it allowed health studies to be submitted as confidential business information, but wrote in a response to questions from The Intercept that it made summaries of the studies public. The agency statement also noted that “EPA is currently following our established process to review these and other submissions and declassify unwarranted CBI claims.”
When asked for comment, Chemtura did not dispute that it claimed the studies as confidential, but said in a statement that “providing information as Confidential Business Information to protect proprietary technical information is in full legal compliance with what is allowed under Federal regulations.” Chemtura also wrote that it strongly disagrees “with the characterization that there is something wrong with confidentiality claims.”
The law allows us to make a claim of confidentiality in order to protect our investments. Companies invest a lot of money in the development and manufacture of its products. This investment comes in many forms: research, physical testing, construction of manufacturing plants, product registrations, toxicology testing, marketing and advertising are among the many investments a company can make. These investments form proprietary information which is a barrier to entry for other companies. Giving away your investments to competitors is an unsustainable business practice for companies who seek to be successful. In the case of toxicology data, competitors have and do use public information they obtain to register competing “copycat” products against the data originators. Technical data is a valuable asset, and care should be taken in how companies distribute that intellectual property.
Gartner worries that the agency’s acceptance of Chemtura’s inappropriate CBI claim — and apparent failure to notice that the EPA itself was violating the law — signals a much bigger problem. “Nobody blinked an eye at EPA,” said Gartner. “It raises a lot of questions. How many other health and safety studies have been submitted to the agency and claimed as CBI?”
It’s impossible to answer Gartner’s question, since the information needed to determine whether a CBI claim is justified is itself often confidential.

Heather Stapleton seals bottles with a liquid sample of foam before testing them for harmful flame retardants at Duke University on April 30, 2012, in Durham, N.C.
Photo: Sara D. Davis/Chicago Tribune/Getty Images
Flame Retardant Dust
Part of the problem is the weakness of the law. TSCA indicates that companies should have to prove that disclosure of the information they’re claiming as CBI would likely “cause substantial harm to the business’s competitive position.” But while the EPA can face hefty fines if it violates a company’s confidentiality, TSCA offers no way to penalize companies that make false confidentiality claims. The EPA has helped companies declassify documents and encourages them to review their confidentiality claims through “the CBI Voluntary Challenge” and, in 11 cases, has disallowed CBI claims, according to an agency spokesperson. But it has never punished a company for a false claim.

Firemaster 550, a flame retardant, is pictured at Heather Stapleton’s lab at Duke University.
Photo: Sara D. Davis/Chicago Tribune/Getty Images
Environmental researchers need to be resourceful — and lucky — to penetrate the obscurity created by CBI. Heather Stapleton, a scientist at Duke University who studies household dust, was able to show that two of the BPC flame retardants were widespread in the environment only because one of her colleagues happened to suggest that a new chemical she noticed in a dust sample might be a component of Firemaster 550, a flame retardant made by Chemtura. Luckily for Stapleton, the colleague happened to have — and share with her — a sample of the product, which isn’t readily available to scientists. Stapleton was able to match the molecules in it to those in the product sample.
Stapleton’s discovery might have ended there. But after giving a talk about her research, a furniture manufacturer who was in the audience gave her a letter from Chemtura saying that the company’s prenatal development studies of its product had found “some effects.” The letter went on to assure the manufacturers that the risk was “negligible,” despite the findings, since the product didn’t leak into the environment.
But Stapleton’s work proved otherwise. The chemical was clearly making its way into the environment if it was showing up in dust samples. Alarmed, she asked Chemtura for its health studies of Firemaster 550. Stapleton said the company declined to supply them (Chemtura told me that it has no record of Stapleton requesting the studies), and so she asked the EPA for any data it had on the product. “They mailed me a CD that had 800 pages and 90 percent was blocked out for CBI,” Stapleton told me recently. “I couldn’t make heads or tails of that document.”
According to a statement from the EPA, the agency declassified the company name, chemical names, and individual ingredients of Firemaster 550 in 2010, and this information is now available in the public docket.
So Stapleton decided to use some of her remaining Firemaster 550 sample to study the product’s health effects, exposing pregnant rats to varying doses of the substance and observing the health of their offspring. She found that exposure could have clear effects on the rats’ babies, which were more likely to become obese and show signs of anxiety. Female rats whose mothers were exposed to Firemaster 550 were more likely to experience early onset of puberty, and males whose mothers were exposed at levels lower than the company deemed safe had an increased rate of heart defects. Stapleton also concluded that Firemaster 550 is an endocrine disruptor.
Substantial Risk Reports
Independent research on the health effects of the replacements for C8 and longer-chain perfluorinated compounds has only recently begun in earnest. But several studies already indicate problems similar to those linked to C8, which include immune disorders, reproductive problems, and two kinds of cancer.
The most worrisome health information comes from industry itself. Chemical manufacturers are required by Section 8 (e) of the Toxic Substances Control Act to report any information to the EPA that “reasonably supports the conclusion that” a substance they make or use “presents a substantial risk of injury to health or the environment.” But the critical information in these 8 (e) reports can also be claimed as confidential. Last year, the Environmental Working Group reviewed more than 100 Section 8 (e) reports that had been submitted for perfluorinated chemicals between 2007 and 2015 and found that, among the 85 percent in which the chemical’s name was withheld, “reported health effects of exposure included death; maternal and developmental toxicity; degeneration and necrosis of the kidneys; chromosome aberrations; changes to the weight of the heart, kidney, liver, thymus, spleen, prostate, ovaries and adrenal glands; lethargy; and irregular breathing.”
The EPA has possessed evidence of the health effects of DuPont’s C8 replacement, GenX, since at least April 2006, when DuPont filed the first of 16 Section 8 (e) reports about the chemical. Some of those reports reference a 2009 consent order, which The Intercept obtained through a Freedom of Information Act request. That document — in which the specific identity of the replacement chemical and a closely related salt molecule, their production volume, manufacturing process and sites, processing, use, and other information have been withheld as CBI — lays out the agency’s many concerns about DuPont’s C8 replacement. It notes, for instance, that it has evidence that the chemical and its salt are toxic to lab animals and cause mutations in mammalian and human cells. The document also lays out concerns that the molecules “will persist in the environment, could bioaccumulate, and be toxic (“PBT”) to people, wild mammals, and birds”; that “there is high concern for possible environmental effects over the long-term”; and that “EPA has human health concerns for the PMN substances.”
An analysis of the 8 (e) reports, which are based on DuPont’s experiments on lab animals, shows that GenX presents some of the very same health problems that C8 does, including changes in the size and weight of animals’ livers and kidneys, alterations to their immune responses and cholesterol levels, weight gain, reproductive problems, and cancer.
In response to inquiries from The Intercept, DuPont declined to comment, noting that GenX is now a product of Chemours. Chemours responded that “extensive safety testing was conducted” on GenX. “Data suggests that it is not a developmental, reproductive, or genetic toxicant, or a human carcinogen.” (See “New Teflon Toxin Causes Cancer in Lab Animals” for the complete text of Chemours’ response.)
Due to CBI claims, it’s impossible to determine the amounts of the new PFCs that are being manufactured and used in the United States. Without this information and with little monitoring of their presence in the environment, exposure levels are similarly indeterminate. DuPont’s filings in Europe estimate production of GenX at between 10 and 100 tons each year. GenX, however, is only one of the company’s new PFCs. Chemours, the chemical company spun off by DuPont in July 2015, has many additional new formulations of surfactants and repellents for use in textiles, firefighting foam, and leather. Other chemical companies have developed their own substitutes. 3M, which supplied C8 to DuPont for many years, uses a product called ADONA. Solvay, Asahi, Dow Corning, and numerous companies in Japan and Europe have also come up with their own formulations. Zhanyun Wang estimates that tens of thousands of tons of fluorinated alternatives are now produced worldwide.
In May 2015, a group of scientists issued the Madrid Statement, which called for limiting production of all perfluorinated chemicals (regardless of the length of their molecules) based on their persistence and toxicity. The scientists noted that little information has been made public about how poisonous the replacement chemicals are to humans or animals, but that longer-chain PFCs have been shown to cause “liver toxicity, disruption of lipid metabolism, the immune and endocrine systems, adverse neurobehavioral effects, neonatal toxicity and death, and tumors in multiple organ systems” in lab animals and are associated with “testicular and kidney cancers, liver malfunction, hypothyroidism, high cholesterol, ulcerative colitis, lower birth weight and size, obesity, decreased immune response to vaccines, and reduced hormone levels and delayed puberty” in people. And a 2014 study in Environmental Research has already linked one of the C6 replacement molecules, PFHxA, with a health problem that does not seem to be linked to other PFCs — a liver disorder known as Gilbert Syndrome.
There is one way these “shorter-chain” variations seem to be better than the originals they’re replacing. Many of them, though not all, remain in the human body for less time. According to one 2011 document from the European Food Safety Authority, 3M reported that the half-life of its chemical ADONA was between 12 and 34 days in the bodies of three workers. In contrast, it takes humans about four years to clear half of the C8 from their bodies. Although it takes months for lab animals to rid themselves of C8, DuPont has claimed that with GenX, “virtually complete elimination from the body occurs in 12-24 hours.”
But as C8 replacements become increasingly ubiquitous, this improvement may be moot. “Even if it stays for just days,” said Wang, a chemical “still has possibility to cause damage.” Because the replacements are already so widespread, he said, “we’ll keep eating them and drinking them, so we’ll have continuous exposure. And if the environmental concentration in food and water keeps going higher because of increased use, then concentrations in our bodies will also go up.”
Asked for comment, 3M provided the following statement: “We believe that these shorter-chain compounds do not present health risks at the levels they are typically found in the environment.”
In terms of how long they’ll persist in the environment, the new chemicals are just as bad as the C8 they’re meant to replace. Like C8, GenX is extremely stable and will likely persist indefinitely. As A. Michael Kaplan, DuPont’s then-director of regulatory affairs, put it in one of the 8 (e) reports the company submitted to the EPA in 2010, “The biodegradation of the test substance was 0%.”
“It will take thousands of years to break down — or maybe longer,” Wang said of GenX. 3M’s ADONA, he said, will also endure indefinitely. “The company claims that this replacement degrades, but actually it doesn’t.” Indeed, most of the new replacement PFCs — or, in the case of the longer-chain molecules, the substances they degrade into — won’t ever break down. “We’re replacing a super-persistent chemical with super-persistent chemicals.”
Resetting the Clock
It took half a century from the introduction of C8 into commercial use for the public to catch on about its dangers. In part because the EPA has yet to issue binding regulation that could require polluters to be held financially responsible for their mess, most of the contamination from that chemical is still in our environment. The earlier flame retardants that BPCs are replacing — and the dangerous chemicals they degrade into — also remain with us.
Now, with the introduction of next-generation replacement chemicals, industry has reset the clock. In addition to the C8 and the phased-out flame retardants in our water, soil, and air, we are being exposed to hundreds of other chemicals, many of which could endure forever.
DuPont referred to C8 as an “essential processing aid.” Chemours, which has inherited DuPont’s PFC business, notes that its newer generation of fluoropolymer resins, manufactured using GenX, is “critically important.” The company website points out that its products are used to provide cable and internet service, more efficient cars, and “insulation for cabling that is essential for safety, security and performance in buildings, data centers, ships and aircraft.”
But while PFCs are used to make some very useful products, they’re also in many others that are not essential, including food packaging, clothing, make-up, workout gear, and outdoor equipment, such as hiking clothes and tents, which means that nature lovers may be unwittingly spreading the contamination to remote places when they travel. Clearly, many if not all of these products could be manufactured without using PFCs.
The American Chemistry Council insists that “flame retardants provide an important layer of fire protection and help save lives.” But as the Chicago Tribune has reported, the trade organization has used phony customer watchdog groups and bogus claims to make the case for the necessity of flame retardants. Not only do the chemicals provide no meaningful protection from fire, as the Tribune’s reporting made clear, they can actually increase smolder propensity, as California officials noted when the state was doing away with its requirement that furniture makers inject the flame retardants into cushions. Some scientists also insist there is no scientific justification for the current practice of putting flame retardants in electronics. The American Chemistry Council did not respond to our requests for comment.
Although it’s technically possible to rid the environment of some PFCs, the process of finding, extracting, and disposing of them is practically out of reach in most of the world. Most countries won’t be able to pay for it, and the few that can, including the U.S., are unlikely to undertake this incredibly difficult and expensive task.
Rewriting the Law on Toxins
This should be the ideal time to be grappling with the enduring impact of unsafe chemicals. Congress is in the midst of revisiting our lax national chemical safety law, the Toxic Substances Control Act, and reform bills have passed both the House and the Senate. But lawmakers have already missed the opportunity to close one gaping legal hole that allows unsafe chemicals to enter the market, since neither of the bills now being considered would require companies to submit specific safety data before new chemicals are approved for use.
Nor does either bill really fix the confidentiality problem. The Senate’s bill would make some improvements on CBI, requiring the EPA to review past and future confidentiality claims that mask a chemical’s identity, as well as at least a quarter of the CBI claims for other types of information. But the House bill does not mandate any CBI review or lay out penalties for companies that make false claims. And in one important respect, TSCA “reform” could be a step backward: The House bill would allow companies to claim chemical identity in health studies as CBI.
As Congress dickers over reconciling the two TSCA reform bills, the regrettable replacements are accumulating all around us. The researchers who have made it their business to chase after those chemicals meanwhile struggle to keep pace. Stapleton, the researcher at Duke, is raising money to conduct a larger version of her experiment with Firemaster 550, which was criticized for its small sample size. Stapleton’s lab at Duke also runs a public testing program so that people can send in foam samples from their furniture to determine whether it contains dangerous flame retardants.
Wang, for his part, has become increasingly frustrated with the lack of awareness of the irreversible PFC contamination. Time, he says, is running out. “We need to reduce the emissions as fast as possible and evaluate whether uses are essential.” To his great frustration, however, most of his colleagues who work with PFCs are still focused on C8.
Strynar and Lindstrom, the EPA researchers in North Carolina, are hoping their discoveries will spur medical researchers to investigate the health effects of the PFCs they discovered in the Cape Fear River. They themselves have begun to work on developing methods to measure the chemicals, and to test methods for removing them from drinking water. Their research will likely continue for years.
_____________________________________
Related:
- New Teflon Toxin Causes Cancer in Lab Animals
- DuPont and the Chemistry of Deception
- The Case Against DuPont
- How DuPont Slipped Past the EPA
- Poisoning the Well: Toxic Firefighting Foam Has Contaminated U.S. Drinking Water
Sharon Lerner – ✉fastlerner@gmail.com
Go to Original – theintercept.com
DISCLAIMER: The statements, views and opinions expressed in pieces republished here are solely those of the authors and do not necessarily represent those of TMS. In accordance with title 17 U.S.C. section 107, this material is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. TMS has no affiliation whatsoever with the originator of this article nor is TMS endorsed or sponsored by the originator. “GO TO ORIGINAL” links are provided as a convenience to our readers and allow for verification of authenticity. However, as originating pages are often updated by their originating host sites, the versions posted may not match the versions our readers view when clicking the “GO TO ORIGINAL” links. This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml. If you wish to use copyrighted material from this site for purposes of your own that go beyond ‘fair use’, you must obtain permission from the copyright owner.