Superbug: “The Annihilator”
TRANSCEND MEMBERS, 4 Oct 2021
Prof Hoosen Vawda – TRANSCEND Media Service
The Final Nemesis of Humanoids[1]
29 Sep 2021 – “Breaking News[2]: I am Halla Mohieddeen[3]. This is Al Jazeera[4] reporting live from DOHA[5] on 01st December 2031. It is 0413 hours GMT. and we interrupt this documentary with the breaking news, just received from our Washington Bureau[6], that the President of the United States[7] has passed away at 0355 hours GMT, in the Walter Reed Military Medical Center[8], hospital, as confirmed by the Whitehouse Press Secretary[9]. The President has succumbed to the Superbug[10] infection. He was treated by a multidisciplinary team of doctors who have been battling to treat the Superbug “X” infection for a week. It is also reported that one of the doctors in the team, is also infected with Superbug “X” infection and is in a critical condition in the same hospital. Al Jazeera will be constantly updating this developing story, as we receive further information from our live news reporters, stationed at the White House[11], in Washington DC[12]. The death toll from the Superbug X presently stands at 5 million and 15 million are critically ill in hospitals throughout the United States. Globally, 25 million people have died, since the Superbug X Pandemic broke out in the United States, in the summer of 2031.”
This Breaking News could well be the closing sequence from a Stephen King[13] movie, in which the Superbug has caused worldwide decimation of humanoids. Unfortunately it is not and could well be the reality in the next ten years, with the progressive emergence of global antibiotic resistance[14] amongst bacteria [15]in humanoids[16].
It is necessary to examine how the antibiotic resistance has emerged since the discovery of this category of medication for the treatment of infections in the human bodies. Indiscriminate use of antibiotics will reach a stage when the infective bacteria will no longer be sensitive to any known antibiotics, either as a single treatment administration plan, or antibiotic polypharmacy, in which multiple antibiotic therapy is used to treat an infective condition in a single patient. The history of antibiotics can be described in two segments: early history and modern history. Most important stage in the evolution of the antibiotics is the discovery of penicillin by Sir Alexander Fleming.[17] Infections are very common and responsible for many diseases adversely affecting human health. These are the communicable diseases[18], as opposed to non-communicable[19] diseases such as Diabetes[20], Hypertension[21] and Coronary Artery Disease[22]. Most of the communicable diseases are caused by bacteria, appreciating that presently, the SARS Cov-2[23] pandemic is caused by a virus, against which antibiotics are not effective, at all. Infections caused by bacteria can be prevented, managed, and treated through anti-bacterial group of compounds known as antibiotics, which literally means “against life”; in this case, against microbes.[24]
Antibiotics can be loosely defined as the variety of substances derived from bacterial sources (microorganisms) which control the growth of or kill other bacteria. However, synthetic antibiotics, usually chemically related to natural antibiotics, have since been produced that accomplish comparable tasks.
Antibiotics can also be classified based on their chemical structure. A similar level of effectiveness, toxicity and side-effects is rendered by the antibiotics of same structural group. Broad spectrum antibiotics are effective against a broad range of microorganisms in comparison to narrow spectrum antibiotics. Bactericidal antibiotics kill the bacteria whereas bacteriostatic antibiotics halt the growth of bacteria.[25]
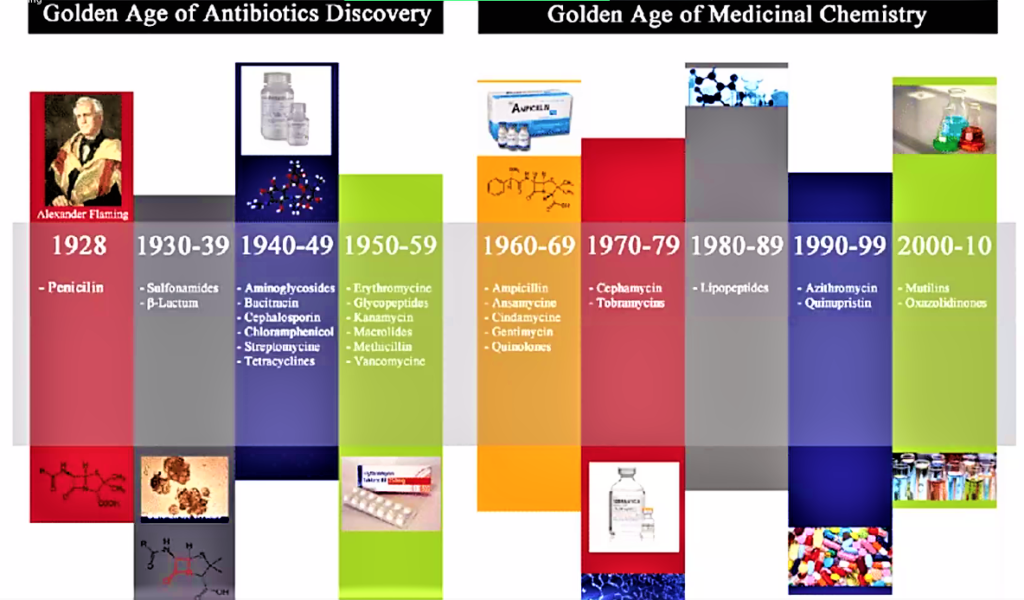
It is interesting to note the history of antibiotics, although not called as such in ancient times, can be described in two segments: early history during ancient times, and the modern history. The Egyptians, during the Pharaonic times[26], used to apply moldy bread to treat infected lesions. During the Islamic Era[27], had some idea about the causative factors in infection and one local leader, wanted to build a hospital. To establish where he must build such a facility, he went around the town hanging large chunks of meat at strategic places. The next day, he went back and concluded that the hospital should be built at the site where the hung meat had the least number of flies on it. The Greeks[28] and Indians[29] used molds and other plants to treat infections. In Serbia[30], moldy bread was traditionally used to treat wounds and infections, as well. Warm soil was used in Russia by peasants to cure infected wounds[31]. Sumerian doctors gave patients beer soup mixed with turtle shells and snake skins. Babylonian doctors healed the eyes using a mixture of frog bile and sour milk. The Sri Lankan army used oil cake[32] (sweetmeat) to server both as desiccant and antibacterial, although the microorganisms, including bacteria were discovered mulch later.
In recent history, in 1640 in England, John Parkington[33] recommended using mold for the treatment in his book on pharmacology. In 1870 in England, Sir John Scott Burdon-Sanderson[34] observed that culture fluid covered with mold did not produce bacteria. In 1871, in England, Joseph Lister experimented with the antibacterial action on human tissue on what he called Penicillium glaucium[35]. In 1875, in England, John Tyndall[36] explained antibacterial action of the Penicillium fungus to the Royal Society. In 1877, in France, Louis Pasteur[37] postulated that bacteria could kill other bacteria (anthrax bacilli). In 1897,in France, Ernest Duchesne[38] healed infected guinea pigs from typhoid using mold (Penicillium glaucium). In 1928 in England, Alexander Fleming[39] discovered enzyme lysozyme and the antibiotic substance penicillin from the fungus Penicillium notatum. In 1932, in Germany, Gerhard Domagk, discovered Sulfonamidochrysoidine (Prontosil).[40] During 1940’s and 50’s streptomycin, chloramphenicol, and tetracycline were discovered and Selman Waksman, in 1942, used the term “antibiotics” to describe the group of “chemicals”, with healing properties in cases of infections of different types in both humanoids as well as animals, including fishes and other edible sea life.[41]
Sir Alexander Fleming, a Scottish biologist, defined new horizons for modern antibiotics with his discoveries of enzyme lysozyme (1921) and the antibiotic substance penicillin (1928). The discovery of penicillin from the fungus Penicillium notatum perfected the treatment of bacterial infections such as, syphilis, gangrene and tuberculosis. He also contributed immensely towards medical sciences with his writings on the subjects of bacteriology, immunology and chemotherapy.[42]
Alexander Fleming was born in Loudon, Scotland on 6 August, 1881 in a farming family. He carried on his schooling at Regent Street Polytechnic after his family moved to London in 1895. He joined St. Mary’s Medical School and became research assistant to renowned Sir Almroth Wright after he qualified with distinction in 1906. He completed his degree (M.B.B.S.) with gold medal in 1908 from the University of London and lectured at St. Mart till 1914. He served as Captain during the World War I and worked in battlefield hospitals in France. After the war he returned to St. Mary in 1918 and got elected Professor of Bacteriology in 1928.
Ironically, the discovery of antibiotics is a case of “”One sometimes finds what one is not looking for” (Sir Alexander Fleming). In the work of Alexander Fleming, it was a case of serendipity or Divine intervention, or simply careless research methodology, leading to the accidentally discovery of penicillin. Upon returning from a holiday in Suffolk in 1928, he noticed that a fungus, Penicillium notatum, had contaminated a culture plate of Staphylococcus bacteria he had accidentally left uncovered.[43] The fungus had created bacteria-free zones wherever it grew on the plate. Fleming isolated and grew the mold in pure culture. He found that P. notatum proved extremely effective even at very low concentrations, preventing Staphylococcus growth even when diluted 800 times, and was less toxic than the disinfectants used at the time. He named it penicillin. After early trials in treating human wounds, collaborations with British pharmaceutical companies ensured that the mass production of penicillin (the antibiotic chemical produced by P. notatum was possible. Following a fire in Boston, Massachusetts, USA, in which nearly 500 people died, many survivors received skin grafts which are liable to infection by Staphylococcus. Treatment with penicillin was hugely successful, and the US government began supporting the mass production of the drug. By D-Day in 1944, penicillin was being widely used to treat troops for infections both in the field and in hospitals throughout Europe. By the end of World War II, penicillin was nicknamed ‘the wonder drug’ and had saved many lives.[44]
He was knighted in 1944 and was given the Nobel Prize in Physiology or Medicine in 1945 for his extraordinary achievements which revolutionised the medical sciences. Various types of antibiotics work in either of the following two ways, a bactericidal antibiotic kills the bacteria generally by either interfering with the formation of the bacterium’s cell wall or its cell contents. Penicillin, daptomycin, fluoroquinolones, metronidazole, nitrofurantoin and co-trimoxazole are some examples of bactericidal antibiotics. A bacteriostatic antibiotic stops bacteria from multiplying by interfering with bacterial protein production, DNA replication, or other aspects of bacterial cellular metabolism. Examples of bacteriostatic antibiotics are tetracyclines, sulphonamides, spectinomycin, trimethoprim, chloramphenicol, macrolides and lincosamides.[45]
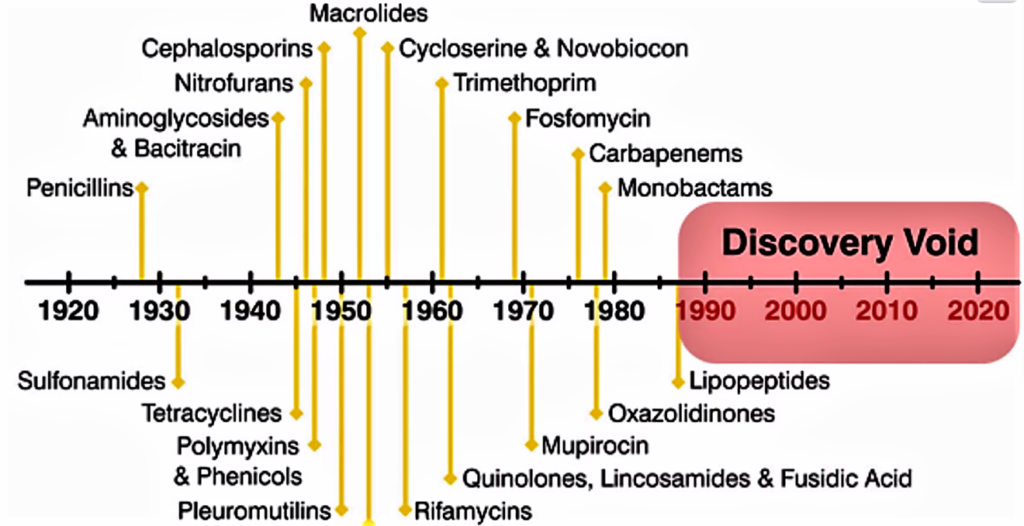
Scientists in Oxford were instrumental in developing the mass production process, and Howard Florey and Ernst Chain shared the 1945 Nobel Prize in Medicine with Alexander Fleming for their role in creating the first mass-produced antibiotic.[46] The introduction of penicillin marked the beginning of the so-called “golden era” of antibiotics which commenced in 1940 and last up to 1962.[47] Most of the antibiotic classes we use as medicines today were discovered and introduced to the market. Each class typically contains several antibiotics that have been discovered over time or are modified versions of previous types. There are for example numerous β-lactams, such as different penicillins and cephalosporins. Lack of new antibiotics. Today, there are very few novel antibiotics under development. At the same time antibiotic resistant bacteria which survives antibiotic treatment are virulent, making available antibiotics ineffective. Thus, we are inevitably facing a major health problem. It is interesting to note that the Big Pharma are not developing novel antibiotics and medicine in general is deplete of antibiotics. One reason for why antibiotic resistant bacteria are attracting so much attention right now is that we have reached a point where there are hardly any treatment alternatives left for some bacterial infections.[48] Resistant infections occurred also in the early days of antibiotic use, but a steady flow of new antibiotics provided alternative treatments. It was possible to simply switch treatment once resistance against a specific antibiotic became a major problem. But then the antibiotics stopped coming. The latest discovery of a new antibiotic class that has reached the market was back in 1987. Since then there has been a lack of innovation in the field, and today there are few novel antibiotic classes in the drug pipeline. The consequences are seen worldwide as bacterial infections are becoming hard to treat, once again. It is of special concern is the lack of antibiotics against Gram-negative bacteria. The attached figure shows when the different major antibiotic classes were discovered.[49]
The question what needs to be raised is why are there so few new antibiotics in development? The reasons are multiple. There are scientific difficulties and it is extremely difficult to develop an antibiotic drug. Firstly. The antibiotic needs to be delivered to the targeted, infected region in the body at a high enough concentration without being toxic to the patient. Then, it also has to enter and stay in the bacterial cell, which has proven very problematic. Efforts to screen large existing libraries of small molecules have failed to find new antibiotics. Financial and regulatory hurdles are further impediments It is extremely expensive and often takes ten years or more to develop a novel antibiotic group. Each new formulation needs to go through rigorous testing for activity and patient safety. Of these molecules investigated, only a minority will actually pass through the entire drug-development process. Resistance development can be fast and may hamper usability, which could result in low profits for the Big Pharma and the shareholders will be extremely unhappy. In addition, novel antibiotics would have to be used sparingly to avoid resistance development. Companies have pointed out regulatory requirements to be unclear, which have led to uncertainty of the likelihood of approval of new drugs. Another reason is that as the population ages, non-communicable diseases such as Alzheimer’s , malignancies and obesity are gaining prominence and the Big Pharma find development of these medication more profitable than antibiotics. Therefore, lack of know-how: poor financial incentives in combination with the technical difficulty to develop new antibiotics have made many pharmaceutical companies scale-down or abandon their antibiotic development programmes. This has resulted in a loss of skills and specialised personnel in the field.
A 2017 report from the World Health Organization (WHO)[50] shows a serious lack of new antibiotics under development. The analysis identifies 51 new antibiotics and biologicals in clinical development to treat priority antibiotic-resistant pathogens, as defined by WHO, such as tuberculosis and Clostridium difficile. Only eight of these drug candidates are classified as truly innovative, highlighting the difficulties in research and development of new antibiotics. Considering a success rate of 14% from Phase I trials to approval, only ten of the substances can be expected to reach the market within 10 years. One or two of these are potentially active against Gram-negative pathogens.
Furthermore, the antibiotic resistance problem cannot be solved, but only managed. In the long term, there needs to be alternative ways to prevent and treat bacterial infections and not rely solely on antibiotics. However, antibiotics will still be needed in the short- to medium-term time frames. It is, therefore important to learn from past mistakes to preserve any new antibiotic that reaches the market and to maintain and possibly enhance what power is left in the older antimicrobials. It is important to understand that the problem of antibiotic resistance cannot be “solved” by the discovery of one or a few new antibiotics. Antibiotic resistance will eventually develop to any antibiotic, but prudent use will slow the process. That is, if a new antibiotic reaches the market, it should be used responsibly, or it will soon be ineffective due to bacterial resistance development. This means that another model than high-volume sales must be found for regaining development costs and making profits for pharmaceutical companies. This process of separating sales volumes from profit is commonly referred to as “delinkage”. Furthermore, different bacterial diseases require different antibiotics. Thus, there needs to be a continuous supply of new antibiotics and a continuous needs analysis by the health sector, collectively.[51]
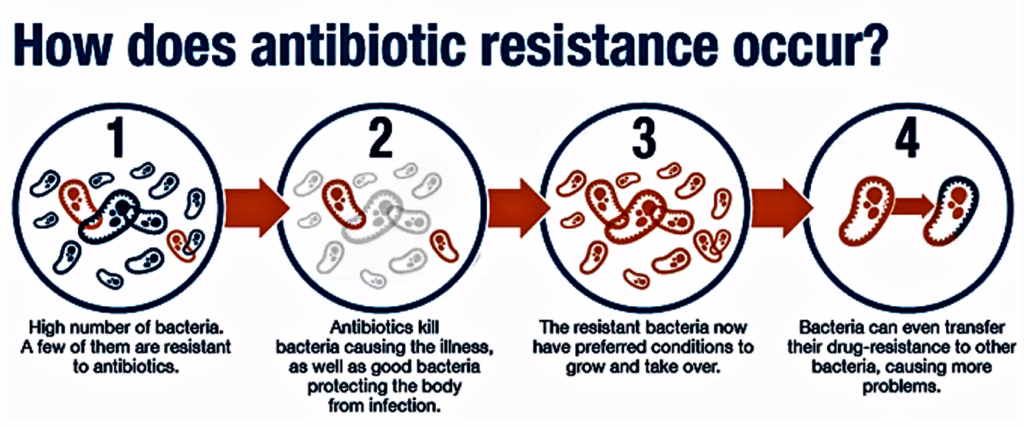
The causes of antibiotic resistance are multifactorial and shown diagrammatically in Figure attached.
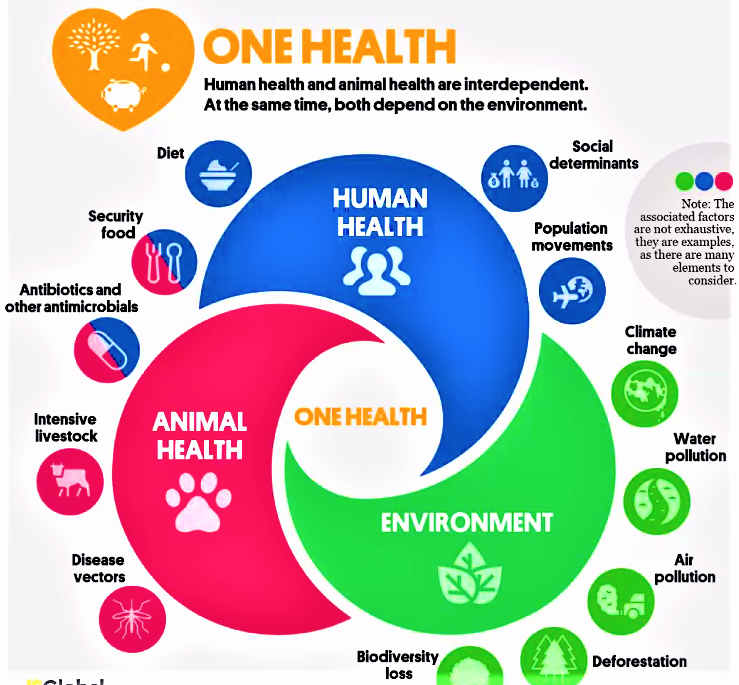
It is to be noted that antibiotics fed to animals as in livestock agriculture and fish farming, such as the lucrative Salmon farms contributes significantly to antibiotic resistance in humanoids. In pursuit of material wealth and improving economies of countries the government have overlooked the close interaction between human health animal health and environmental health as illustrated in Figure. Human Health and Animal Health are interdependent and simultaneously they both depend on environmental health.
Hence, Global Climatic change will impact on animal health, to a greater extent on animal health, whereby disease will become prevalent in animals, requiring greater use of synthetic antibiotics causing greater resistance to antibiotics in humanoids in the future. It is imperative that governments who are indifferent towards climate change must be accountable for adding to carbon emissions and indirectly impacting upon the progress of untethered antibiotic resistance in humans. This important concept to ensure the continued survival and wellbeing of humanoids for the core basis of “One Health”[52], Health for All, which, most regrettably closed doors in 2021 after 15 years of working hard to further their mission to stop the number one killer presently, which is the rising incidences of chronic diseases in vulnerable communities in Australia and internationally. Concomitant with this is the rising rates of increasingly potent antibiotics to treat one of the most common lifestyle disease, Chronic Bronchitis and related chronic pulmonary diseases such as Bronchiectasis. Adding the the greatl to the burden of diseases in the economically hamstrung, South Africa, due to environmental pollution as in the city of New Delhi in India and elsewhere, experienced recently. The greater the antibiotic usage the greater is the development of resistance making a particular antibiotic totally ineffective in a few years after launch. This trend is certainly aggravated by incorrectly treating Primary Covid Pneumonitis with poly-antibiotic therapy in South Africa, at least, where concomitant infections with HIV and Pulmonary Tuberculosis is common constitute the “Unholy Triad” of HIV, TB, SARS Cov-2 and the emerging Antibiotic Resistance, rapidly.
The Bottom Line is that from the time of Sir Alexander Fleming’s drastically changing the fate of the world in a petri dish[53] today the future of humanoids depend on curtailing the indiscriminate use of antibiotics, resulting in antimicrobial resistance. The current SARS Cov-2 pandemic has caused physicians routinely prescribing potent antibiotics such as Azithromycin[54] and Ceftriaxone[55] in patients with pulmonary involvement and this will further add to the ever-increasing spectre of antibiotic resistance, in humanoids. The problem is that the bacterial microbes are ever mutating by altering their genetic mechanism to survive in the human hosts, with deadly consequences. It is the case of the traditional hypothesis of Charles Darwin’s concept of survival of the fittest, whereby the bacteria will become more powerful than the pharmacological armamentarium available to medical science to enable the healthcare professionals to contain and treat infections of eternally increasing potency in humans. In the end there will be no available antibiotic to treat the Super, Superbug which humanoids have given the bacteria ample opportunities since 1928, to the post SARS Cov-2 era, to effectively develop by constantly modifying its DNA structure to resists all presently know antibiotics. Our extinction is guaranteed by the very antibiotics we created way back in1928 to help humanity in sickness, will ironically be the cause of the doom of humankind, by the emergence of a new global Superbug Pandemic, causing, millions of human fatalities. To prevent this scenario from unfolding, there has to be a drastic paradigm mind shift, amongst the Big Pharma, who are financially rewarded for their antibiotic innovations, the Healthcare Professionals, some of whom are “attending to and treating 200 patients a day”, by their own admissions, the Hospitals, the Primary Healthcare Clinics, the recently qualified, enthusiastic young doctors and nurses, the end user: the patients and the numerous global committees in charge of antibiotic stewardship, established by concerned institutes, including the World Health Organisation[56]. In fact the situation is so desperate that an intern rotating through Internal Medicine, in Durban, South Africa, informed the author that “ All patients in Internal Medicine, irrespective of the diagnosis, need to be on Ceftriaxone”[57] We all must inculcate a culture of collective responsibly in curtailing the rampant prescription of increasingly potent antibiotics in terms of infection prevention, mitigation and control, if we need to ensure our continued existence on planet Earth in the future, for at present the projected future is dismal for humanoids.
References:
[1] https://desecrateethereal.bandcamp.com/album/the-inexorable-nemesis
[2] https://www.thoughtco.com/what-is-a-breaking-news-story-2073757#:~:text=Breaking%20news%20refers%20to%20events%20that%20are%20currently,a%20fire%2C%20a%20tornado%20%E2%80%94it%20could%20be%20anything.
[3] https://en.wikipedia.org/wiki/Halla_Mohieddeen
[4] https://www.google.com/search?q=Al+Jazeera&rlz=1C1PNBB_enZA933ZA933&oq=Al+Jazeera&aqs=chrome..69i57j69i65.6098745j0j7&sourceid=chrome&ie=UTF-8#:~:text=Al%20Jazeera%20English%20is%20a%20television%20news%20channel%20broadcast%20to%20the%20world%20by%20the%20Al%20Jazeera%20Media%20Network.%20It%20is%20the%20first%20English-language%20news%20channel%20to%20be%20headquartered%20in%20the%20Middle%20East.%20Instead%20of%20being%20run%20centrally%2C%20news%20management
[5] https://www.bing.com/travelguide?q=Doha&l2sid=c5b0d5d3-8bd3-bd8d-1f71-c25a489a1a0d&form=PLACAB
[6] http://america.aljazeera.com/profiles/b/washington-d-c-bureau.html
[7] https://www.bing.com/search?q=president+of+the+united+states+wikipedia&qs=LT&pq=president+of+the+united+states+&sk=LT4&sc=6-31&cvid=CADE9F57290B414A8516426ADC9B7D2B&FORM=QBRE&sp=5#:~:text=The%20president%20of%20the%20United%20States%20is%20the%20head%20of%20state%20and%20head%20of%20government%20of%20the%20United%20States%20of%20America.%20The%20president%20directs%20the%20executive%20branch%20of%20the
[8]https://en.wikipedia.org/wiki/Walter_Reed_National_Military_Medical_Center
[9] https://www.bing.com/search?q=Whitehouse+spokesperson&qs=n&form=QBRE&msbsrank=6_7__0&sp=-1&pq=whitehouse+spokesperson&sc=7-23&sk=&cvid=2A38C3ECB7944864A3752B3CF9660DB2#:~:text=White%20House%20Official-,The%20White%20House%20press%20secretary%20is%20a%20senior%20White%20House%20official%20whose%20primary%20responsibility%20is%20to%20act%20as%20spokesperson%20for%20the%20executive%20branch%20of%20the%20federal%20government%20of%20the%20United%20States%2C%20especially%20with%20regard%20to%20the%20president,-%2C%20senior%20aides%20and
[10] https://www.mayoclinic.org/diseases-conditions/infectious-diseases/expert-answers/superbugs/faq-20129283
[12] https://www.bing.com/travelguide?q=Washington%2c+D.C.&l2sid=216726d1-8987-06d3-5eff-823da05c3d3c&form=PLACAB
[13] https://www.shortform.com/blog/stephen-kings-writing-process/
[14] https://www.bing.com/search?q=antibiotic+resistance+definition&qs=CT&pq=antibiotic+resistance+&sc=6-22&cvid=F1B49FB350EA424996B3E34C6F1C1302&FORM=QBRE&sp=1#:~:text=Antimicrobial%20resistance%20occurs,to%20as%20%E2%80%9Csuperbugs%E2%80%9D.
[15]https://www.bing.com/search?q=bacteria+definition&qs=AS&pq=bacteria+&sc=6-9&cvid=C7F5D4B924EA49B4A53F556018F458A4&FORM=QBRE&sp=1#:~:text=All%20images-,Bacteria%20are%20ubiquitous%2C%20mostly%20free-living%20organisms%20often%20consisting%20of%20one%20biological%20cell.%20They%20constitute%20a%20large%20domain%20of%20prokaryotic%20microorganisms,-.%20Typically%20a%20few
[16] https://en.wikipedia.org/wiki/Humanoid#:~:text=A%20humanoid%20(/%CB%88hju%CB%90m%C9%99n%C9%94%C9%AAd,identical%2C%20to%20those
[17] https://explorable.com/history-of-antibiotics#:~:text=History%20of%20antibiotics%20can%20be%20described%20in%20two%20segments%20early%20history%20and%20modern%20history.%20Most%20important%20is%20the%20discovery%20of%20pencillin%20by%20Alexander%20Fleming.
[18] https://www.merriam-webster.com/dictionary/communicable%20disease
[19] https://www.bing.com/search?q=communicable+diseases+definition&qs=CT&pq=communicable+diseases&sk=AS1&sc=6-21&cvid=E8EDFE0D92CD41538B54A0BBB6914F20&FORM=QBRE&sp=2
[20] https://www.bing.com/search?q=diabetes&qs=AS&pq=diabetes&sc=6-8&cvid=7648F4829C2F452EA887A7BE7620D5F7&FORM=QBRE&sp=1#:~:text=Diabetes%20mellitus%2C%20commonly%20known%20as%20just%20diabetes%2C%20is%20a%20group%20of%20metabolic%20disorders%20characterized%20by%20a%20high%20blood%20sugar%20level%20over%20a%20prolonged%20period%20of%20time.%20Symptoms
[21] https://www.bing.com/search?q=hypertension+definition&qs=EP&pq=hypertension&sk=EP1&sc=6-12&cvid=5ED320D6067D43D2B38A3129531F9F39&FORM=QBRE&sp=2#:~:text=Hypertension-,Hypertension%20(HTN%20or%20HT)%2C%20also%20known%20as%20high%20blood%20pressure%20(HBP)%2C%20is%20a%20long-term%20medical%20condition%20in%20which%20the%20blood%20pressure%20in%20the%20arteries%20is%20persistently%20elevated.%20High%20blood,-pressure%20typically%20does
[22] https://www.bing.com/search?q=Cardiovascular+disease&filters=ufn%3a%22Cardiovascular+disease%22+sid%3a%220e9f68f1-a5bd-abb6-311d-8167cc522a81%22&FORM=SNAPST#:~:text=Cardiovascular%20disease%20(CVD)%20is%20a%20class%20of%20diseases%20that%20involve%20the%20heart%20or%20blood%20vessels.%20CVD%20includes%20coronary%20artery%20diseases%20(CAD)%20such%20as%20angina%20and%20myocardial%20infarction%20(commonly%20known%20as%20a%20heart%20attack).%20Other%20CVDs%20include
[23] https://www.bing.com/search?q=SARS+Cov-2+&form=ANNTH1&refig=715da51c57784b198f5204d1159461e4#:~:text=virus%20that%20causes%20COVID-19%20(coronavirus%20disease%202019)%2C%20the%20respiratory%20illness%20responsible%20for%20the%20COVID-19%20pandemic.%20Also%20colloquially%20known%20simply%20as%20the%20coronavirus%2C%20it%20was%20previously%20referred%20to%20by%20its%20provisional
[24] https://www.bing.com/search?q=history+of+antibiotics&cvid=4cbbb4ff085b4a75ad0e7e42268d9801&aqs=edge.0.0l5.11112j0j1&pglt=43&FORM=ANNTA1&PC=U531#:~:text=Problems%20with%20Antibiotics-,The%20term%20antibiotics%20literally%20means%20%E2%80%9Cagainst%20life%E2%80%9D%3B%20in%20this%20case%2C%20against%20microbes,-.%20There%20are%20many
[25] https://explorable.com/history-of-antibiotics
[26] https://www.historymuseum.ca/cmc/exhibitions/civil/egypt/egctimee.html
[27] http://en.wikipedia.org/wiki/Islamic_Golden_Age
[28] https://www.restaurantnorman.com/how-did-ancient-greeks-treat-illnesses/
[29] https://www.bing.com/search?q=How+did+the+indians+treat+infection&qs=n&form=QBRE&msbsrank=0_0__0&sp=-1&pq=how+did+the+indians+treat+infection&sc=0-35&sk=&cvid=7A8CC57EB94B4FBAB70E9B872C39178D#:~:text=Answer%20by%20Drew%20Smith%2C%20Former%20R%26D%20director%20at%20SomaLogic%20and%20MicroPhage%2C%20on%20Quora%3A%20It%20is%20certain%20that%20people%20have%20always%20treated%20diseases%2C%20including%20infections%2C%20with%20some%20kind%20of%20pharmacology
[30] https://vkool.com/how-to-treat-bacterial-infection/
[31] https://www.bing.com/search?q=Warm+soil+was+used+in+Russia+by+peasants+to+cure+infected+wounds&qs=n&form=QBRE&msbsrank=0_1__0&sp=-1&pq=warm+soil+was+used+in+russia+by+peasants+to+cure+infected+wounds&sc=1-64&sk=&cvid=10E8B0DA39E34416AD4D5FEA3F7C9C55#:~:text=Date-,Warm%20soil%20was%20used%20in%20Russia%20by%20peasants%20to%20cure%20infected%20wounds.%20Sumerian%20doctors%20gave%20patients%20beer%20soup%20mixed%20with%20turtle%20shells%20and%20snake%20skins.%20Babylonian%20doctors%20healed%20the%20eyes%20using%20a%20mixture,-of%20frog%20bile
[32] https://explorable.com/printpdf/history-of-antibiotics
[33] https://www.nosterprobiotics.com/history-of-antibiotics/
[34] https://en.wikipedia.org/wiki/John_Scott_Burdon-Sanderson
[35] https://www.bing.com/search?q=1871%2C+in+England%2C+Joseph+Lister+experimented+with+the+antibacterial+action+on+human+tissue+on+what+he+called+Penicillium+glaucium&qs=n&form=QBRE&msbsrank=0_1__0&sp=-1&pq=1871%2C+in+england%2C+joseph+lister+experimented+with+the+antibacterial+action+on+human+tissue+on+what+he+called+penicillium+glaucium&sc=1-129&sk=&cvid=9839AE15E832406CB70887F53ED4E4A3#:~:text=Sanderson%27s%20discovery%20prompted%20Joseph%20Lister%2C%20an%20English%20surgeon%20and%20the%20father%20of%20modern%20antisepsis%2C%20to%20discover%20in%201871%20that%20urine%20samples%20contaminated%20with%20mould%20also%20did%20not%20permit%20the%20growth%20of%20bacteria.%20Lister%20also%20described%20the%20antibacterial
[36] https://snaccooperative.org/ark:/99166/w6w37zm7
[37] https://en.wikipedia.org/wiki/L._Pasteur
[38] https://www.bing.com/search?q=In+1897%2Cin+France%2C+Ernest+Duchesne&qs=n&form=QBRE&msbsrank=0_1__0&sp=-1&pq=in+1897%2Cin+france%2C+ernest+duchesne&sc=1-34&sk=&cvid=342AC1B786764AD89783C98A095BD120#:~:text=1897%2C%20Ernest%20Duchesne%20(figure)%2C%20a%2023-year-old%20medical%20student%2C%20presented%20and%20defended%20his%20thesis%20entitled%20Contribution%20to%20the%20study%20of%20vital%20competition%20between
[39] https://www.bing.com/search?q=1928+in+England%2C+Alexander+Fleming+&qs=n&form=QBRE&msbsrank=0_1__0&sp=-1&pq=1928+in+england%2C+alexander+fleming+&sc=1-35&sk=&cvid=8E40EB83A2BC483AB2C352B9BFE21C0A#:~:text=1928%2C%20Sir%20Alexander%20Fleming%20was%20studying%20Staphylococcus%20bacteria%20growing%20in%20culture%20dishes.%20He%20noticed%20that%20a%20mold%20called%20Penicillium%20was%20also%20growing%20in%20some%20of%20the%20dishes.%20A%20clear%20area%20existed%20around%20the%20mold%20because%20all%20the%20bacteria
[40] https://www.bing.com/search?q=1932%2C+in+Germany%2C+Gerhard+Domagk%2C+discovered+Sulfonamidochrysoidine+%28Prontosil%29.&qs=n&form=QBRE&msbsrank=0_1__0&sp=-1&pq=1932%2C+in+germany%2C+gerhard+domagk%2C+discovered+sulfonamidochrysoidine+%28prontosil%29.&sc=1-80&sk=&cvid=2222BE30427B43B0B78EBFB74B9BE83C#:~:text=an.%20Kauf%20Bunter!-,Gerhard%20Domagk%2C%20(born%20October%2030%2C%201895%2C%20Lagow%2C%20Brandenburg%2C%20Germany%E2%80%94died%20April%2024%2C%201964%2C%20Burgberg%2C%20near%20K%C3%B6nigsfeld%2C%20West%20Germany%20%5Bnow%20in%20Germany%5D)%2C%20German%20bacteriologist%20and%20pathologist%20who%20was%20awarded%20the%201939%20Nobel%20Prize%20for%20Physiology,-or%20Medicine%20for
[41] https://www.scribd.com/document/439386768/3-SEM-BCOM-SCIENCE-AND-SOCIETY
[42] https://en.wikipedia.org/wiki/Alexander_Fleming
[43] https://microbiologysociety.org/members-outreach-resources/outreach-resources/antibiotics-unearthed/antibiotics-and-antibiotic-resistance/the-history-of-antibiotics.html#:~:text=Alexander%20Fleming%20was,at%20the%20time.
[44] https://www.bing.com/search?q=By+D-Day+in+1944%2C+penicillin+was+being+widely+used+to+treat+troops+for+infections+both+in+the+field+and+in+hospitals+throughout+Europe.+By+the+end+of+World+War+II%2C+penicillin+was+nicknamed+%27the+wonder+drug%27+and+had+saved+many+lives&qs=n&form=QBRE&msbsrank=0_1__0&sp=-1&pq=by+d-day+in+1944%2C+penicillin+was+being+widely+used+to+treat+troops+for+infections+both+in+the+field+and+in+hospitals+throughout+europe.+by+the+end+of+world+war+ii%2C+penicillin+was+nicknamed+%27the+wonder+drug%27+and+had+saved+many+lives&sc=1-231&sk=&cvid=A6429C9612464B2E80F902555F783AEB#:~:text=Treatment%20with%20penicillin%20was%20hugely%20successful%2C%20and%20the%20US%20government%20began%20supporting%20the%20mass%20production%20of%20the%20drug.%20By%20D-Day%20in%201944%2C%20penicillin%20was%20being%20widely%20used%20to%20treat%20troops%20for%20infections%20both%20in%20the%20field%20and%20in%20hospitals%20throughout
[45] https://explorable.com/history-of-antibiotics
[46] https://microbiologysociety.org/members-outreach-resources/outreach-resources/antibiotics-unearthed/antibiotics-and-antibiotic-resistance/the-history-of-antibiotics.html
[47] https://www.reactgroup.org/toolbox/understand/antibiotics/development-of-antibiotics-as-medicines/#:~:text=The%20introduction%20of,penicillins%20and%20cephalosporins.
[48] https://www.reactgroup.org/toolbox/understand/how-did-we-end-up-here/few-antibiotics-under-development/#:~:text=We%20are%20running%20out%20of%20antibiotics.%20One%20reason%20for%20why%20antibiotic%20resistant%20bacteria%20are%20attracting%20so%20much%20attention%20right%20now%20is%20that%20we%20have%20reached%20a%20point%20where%20there%20are%20hardly%20any%20treatment%20alternatives%20left%20for%20some%20bacterial%20infections.
[49] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3109405/
[50] https://www.reactgroup.org/toolbox/understand/how-did-we-end-up-here/few-antibiotics-under-development/#:~:text=Why%20so%20few,Gram-negative%20pathogens.
[51] https://www.reactgroup.org/toolbox/understand/how-did-we-end-up-here/few-antibiotics-under-development/
[52] https://onehealthorganisation.org/#:~:text=One%20Health%20closed%20doors%20in%202021%20after%2015%20years%20of%20working%20hard%20to%20further%20our%20mission%20to%20stop%20the%20number%20one%20killer%20today%20%E2%80%93%20the%20rising%20chronic%20disease%20rates%20in%20vulnerable%20communities%20in%20Australia%20and%20internationally.
[53] https://www.thegreatcoursesdaily.com/a-brief-history-of-antibiotics-from-penicillin-to-modern-day-medicine/
[54] https://www.medicalnewstoday.com/articles/azithromycin-oral-tablet
[55] http://reference.medscape.com/drug/rocephin-ceftriaxone-342510
[56] https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf
[57] Personal communication to the author by a newly qualified intern, July 2021.
______________________________________________
 Professor G. Hoosen M. Vawda (Bsc; MBChB; PhD.Wits) is a member of the TRANSCEND Network for Peace Development Environment.
Professor G. Hoosen M. Vawda (Bsc; MBChB; PhD.Wits) is a member of the TRANSCEND Network for Peace Development Environment.
Director: Glastonbury Medical Research Centre; Community Health and Indigent Programme Services; Body Donor Foundation SA.
Principal Investigator: Multinational Clinical Trials
Consultant: Medical and General Research Ethics; Internal Medicine and Clinical Psychiatry:UKZN, Nelson R. Mandela School of Medicine
Executive Member: Inter Religious Council KZN SA
Public Liaison: Medical Misadventures
Activism: Justice for All
Email: vawda@ukzn.ac.za
Tags: Antibiotics, Science and Medicine
This article originally appeared on Transcend Media Service (TMS) on 4 Oct 2021.
Anticopyright: Editorials and articles originated on TMS may be freely reprinted, disseminated, translated and used as background material, provided an acknowledgement and link to the source, TMS: Superbug: “The Annihilator”, is included. Thank you.
If you enjoyed this article, please donate to TMS to join the growing list of TMS Supporters.

This work is licensed under a CC BY-NC 4.0 License.